Properties, Synthesis and reactivity of 7-Azaindoles
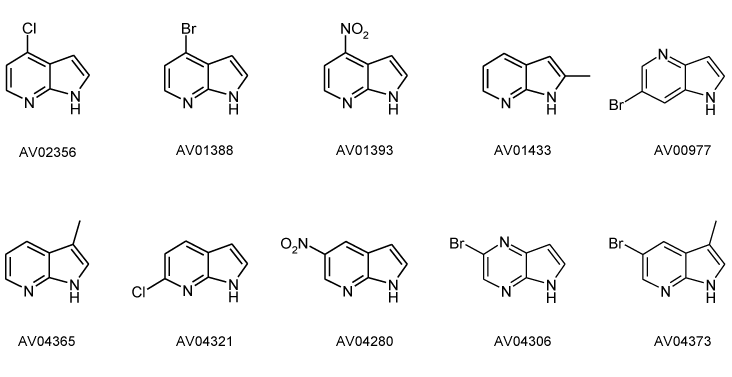
7-Azaindole or 1-H-Pyrrolo [2,3-b] pyridine or 1,7-Diazaindene is an organic compound whose empirical formula is C7H6N2. It is a heterocyclic aromatic compound. It has bi-cyclic structure, is consisting a Pyrrole ring fused to a Pyridine ring. Its other close relatives are 4-Azaindoles, 5-Azaindoles, 6-Azaindoles, and other 7-Azaindoles.
The properties of 7-Azaindoles are governed by the two N-heteroatomic rings with opposite π-electron effects. 7-Azaindoles are more basic than the Indoles. 7-Azaindoles have been extensively investigated for uses in biological probes and imaging. The 7-Azaindoles being bioisosteres (A medicinal chemistry term; bioisosteres are chemical substituent or groups with similar chemical and physical properties which generate largely alike biological properties to a new chemical compound.) as Indoles, these have fueled significant synthetic interests.
Some properties of the 7-Azaindole are observed as follows: Different types of 7-Azaindole derivatives have shown a different effect on the different species of bacteria, plants, and enzymes etc.
- Stimulation of the vacuolation of the protoplasm in the onion.
- Inhibition of the growth of cultivated tobacco and wild carrot.
- Testified as a potential agrochemical.
- Binding to the enzyme Erm that confers resistance to the antibiotic because of the observed biological activities in enzymes.
- Decreasing the growth of a specific free living Ciliates.
- Application as a potential pharmaceutical agent.
Theoretical studies that were carried out on 7-Azaindoles with the use of a SCF-CI π-electron semi-empirical calculation method have shown the behavior of Nitrogen atom of pyridine ring as a π acceptor and σ acceptor. Also, a more latest study which used ab initio approach has indicated the preference of lower negative charge on the Nitrogen atom of Pyrrolo ring and the highest electron density was observed to be on carbon atom at position three or C3 carbon of the 7-Aziandoles.
Synthesis of 7-Azaindoles:
7-Azaindoles have been the target of extensive efforts as an outcome of their powerful pharmacological activities. The most general method for the construction of the ring system absorbs Pyrrolo annelation into a performed pyridine ring.
- From 2-Aminopyridine derivatives
- Fischer type reactions
- Diels-Alder reactions
- Palladium-Catalyzed reactions
- Synthesis from Pyrrole
Reactivity of 7-Azaindoles: In the period of 1955-59, Robinson and his coworkers published their paper on the reactivity of 7-Azaindoles with many different compounds. Following are some examples to describe the reactivity of 7-azaindole with electrophilic reagents and its fundamental reactions.
- N-Alkylation
- Glycosylation
- Electrophilic reactions like Halogenation, Acylation, Nitration, Sulphur and seleno derivatives etc.
- 3-formyl-7-Azaindole
- 7-Azaindolinones
- N-Amination
Pharmacological properties of different 7-Azaindole derivatives: Different 7-Azaindoles have different pharmacological effects. Some of them are listed below.
- Inhibition of protein kinase C.
- As a Renin inhibitor.
- Activity in Central nervous system and show a resemblance for serotonin receptor.
- As a potential dopaminergic D4 ligand.
- As a potential thrombin inhibitor.
- As an inhibitor of cholesterol biosynthesis.
- Demonstration as a hypolipidemic activity.
- Enzyme inhibition.
- Decreased the smooth muscle proliferation.
Due to their much use in pharmacology, now 7-Azaindoles are being manufactured in industries by many chemical manufacturers. New significant improvements have been obtained in the synthesis of 7-azaindole derivatives notably by the development of palladium-catalyzed reactions and anionic condensation reactions.